Scientific Foundation
Built on a solid knowledge platform
Xinnate is built on more than 15 years of world class research by Professor Artur Schmidtchen and his team at Lund University, working on understanding the natural healing process and why some wounds heal while others don’t. During this work, multifunctional peptides derived from thrombin (TCPs), playing a key role in healing, where discovered, characterized and published in some of the best journals of the world. Xinnate operates in close cooperation with Professor Schmidtchen with the aim to further develop and commercialize proprietary novel thrombin-derived peptides in the areas of wounds, inflammatory skin diseases, surgery and biomaterials.
Our lead candidate drug, TCP-25 Gel, is based on one of these multifunctional TCPs – TCP-25. The mode of action is well investigated and understood, and there is a proven therapeutic effect of TCP-25 Gel on infection and healing.

Professor Artur Schmidtchen
The Modes of action
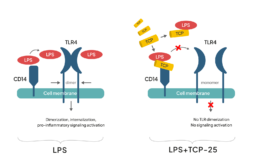
TCP-25 binds specifically to and neutralizes immune stimulating products released from microorganisms (i.e. PAMPs), such as LPS, LTA, peptidoglycan, lipid A, resulting in a reduction of the strong inflammatory response caused by these molecules.TCP-25 also binds CD14, a receptor for PAMPs expressed on innate immune cells (e.g. macrophages), forming a C-shaped structure which blocks the PAMP-binding site of CD14, preventing dimerization of toll-like receptors (TLR) and inflammatory signalling. This leads to down-regulation of excessive levels of cytokines and chemokines (e.g. TNF-ɑ, IL-6, IL-8, MCP-1 and IL-1ß) in wounds, thus promoting normal healing.
TCP-25 has a direct antimicrobial effect by interacting with bacterial and fungal membranes, leading to membrane disintegration, permeabilization and microbial killing.Furthermore, since TCP-25 does not interfere with bacterial phagocytosis, it’s usage leads to retained phagocytic clearance and reduced bacteria-induced inflammation.
The proprietary gel formulation developed for TCP-25 Gel preserves the multimodal action of TCP-25.
Results
An extensive preclinical development package has been finalized with reassuring results supporting clinical development of TCP-25 Gel. Based on this, TCP-25 Gel entered clinical development with a completed phase 1 study demonstrating its safety and preliminary efficacy in healthy volunteers with induced wounds, patients with large complex wounds (VLU) and patients with Epidermolysis bullosa (EB) wounds.
The efficacy of TCP-25 Gel has been demonstrated in relevant animal wound models of bacterial infection and inflammation and proven to be superior to widely used benchmarks containing PHMB or silver.
The unique mode of action of TCP-25 with multiple effects, discriminates the treatment from other existing wound healing products:
Anti-infective

For prevention and early treatment of infections. It is effective on both gram-positive and gram-negative bacteria and also on drug resistant bacteria.
Anti-inflammatory

Naturally managing the paradox keeping the initial healthy level of inflammation and quenching the excessive damaging systemic. No drug with a similar modulatory effect exists today.
Anticoagulative

By inhibiting local contact and tissue factor activation TCP-25 reduces injury-induced harmful inflammation.
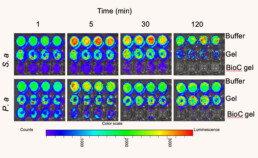
Direct effects of TCP-25 Gel on bacteria
In vitro antibacterial effects of TCP-25 gel.
Bioluminescent S. aureus or P. aeruginosa bacteria were incubated with TCP-25 Gel and the bioluminescent signal was analyzed using bioimaging (IVIS Spectrum).
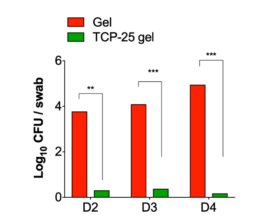
Effects of TCP-25 Gel in a wound mode
TCP-25 Gel reduces infection in wounds.
Microbiological analysis of wounds from days 2, 3, and 4 of treatment.
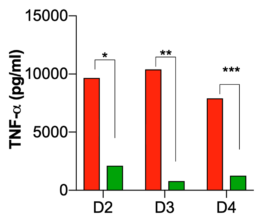
TCP-25 Gel reduces inflammation in wounds.
Analysis of wound fluid cytokines collected on days 2, 3, and 4 of treatment.
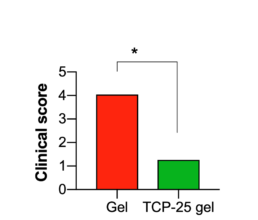
TCP-25 Gel improves wound healing.
Clinical scoring of wounds after 3 days of treatment.
Intellectual property rights
The product candidates are protected by several international patents. The current patent portfolio comprises two patent families. Patent family 1 (TCP-25/P7834) comprises a US patent and an EP patent (in force in Germany, France, the UK and Switzerland/Lichtenstein) claiming the TCP-25 peptide and variants as well as products comprising these peptides and methods of using the peptides. Patent family 2 (TCP-25/10144) also comprises a US patent and an EP patent and claims the medical use of a peptide comprising or consisting of the TCP-25 peptide for use in treating various coagulative disorders, and additionally inflammation. Patent applications on TCP-25 formulations and its use for treatment of Epidermolysis bullosa have recently been filed. The IP-strategy includes to further extend the patent portfolio geographically and temporally.
